Anomalous expansion of water pdf Manawatu-Wanganui
Properties of water Wikipedia Water has a very high specific heat capacity of 4.1814 J/(g·K) at 25 °C – the second highest among all the heteroatomic species (after ammonia), as well as a high heat of vaporization (40.65 kJ/mol or 2257 kJ/kg at the normal boiling point), both of which are a result of …
Water and anomalous liquids
SCIENCE (52) PHYSICS SCIENCE Paper1. The anomalous expansion characteristics of liquid water are crucial to many biological systems. Rather than an approximately constant value for the coefficient of volume expansion, the value for water changes drastically, as illustrated in the figure., Evidence of anomalous thermal expansion of water in cement paste. A comparative study of permeability measurement by thermopermeametry (TPA) and beam bending was performed on cement paste..
water in C−S−H exhibits anomalous dynamics.3 Such anomalous behavior can result in changes in thermal deformation behavior, which can have significant practical implications. For example, C−S−H, serves as the main binding agent present in concrete formed from the reaction of ordinary portland cement (OPC) with water.15,16 Controlling the Water has a very high specific heat capacity of 4.1814 J/(g·K) at 25 °C – the second highest among all the heteroatomic species (after ammonia), as well as a high heat of vaporization (40.65 kJ/mol or 2257 kJ/kg at the normal boiling point), both of which are a result of …
It has been suggested that polywater should have been dismissed on theoretical grounds. The laws of thermodynamics predicted that, since polywater had a higher boiling point than ordinary water, it meant it was more stable, and thus all of Earth's water should have … anomalous expansion of water. The procedure involves monitoring the volume of water below and above the anomalous temperature including some interesting observations such as the super-cooled state of water. The temperature at which water reaches its maximum density is estimated in the experiment.
8/8/2014В В· Water's anomalous behavior is caused by the shape of its molecule and by how its molecules bond to one another. Each water molecule consists of two hydrogen atoms bonded to one oxygen atom (H 2 O). Since hydrogen is electro positive then it is slightly positively charged while oxygen is electro negative hence slightly negatively charged. A beam-bending method that was developed to study the permeability of porous bodies has been used to measure the relative viscosity of salt solutions to water inside the silica pores. This work has demonstrated that water when confined in nanopores shows anomalous behavior and its thermal expansion is higher than bulk water.
8/8/2014В В· Water's anomalous behavior is caused by the shape of its molecule and by how its molecules bond to one another. Each water molecule consists of two hydrogen atoms bonded to one oxygen atom (H 2 O). Since hydrogen is electro positive then it is slightly positively charged while oxygen is electro negative hence slightly negatively charged. Anomalous Properties of Water Property Comparison with other substances Importance in physical-biological environment Heat capacity Highest of all solids and liquids (except NH3) Prevents extreme temperature fluctuations Maintains uniform body temperatures. Latent heat of fusion Highest (except NH3) Thermostatic effect at freezing point owing to
5/2/1991 · It is shown that the thermal expansion coefficient of bound water is different from bulk water. The pH dependence can be explained by increased hydration of side chains at lower pH. The amount in volume of hydration water in a typical protein–water system varies from 0.16 to 0.7. Read "Evidence of anomalous thermal expansion of water in cement paste, Cement and Concrete Research" on DeepDyve, the largest online rental service for scholarly research with thousands of academic publications available at your fingertips.
What is an anomalous expansion of water? Learn about this property of water, along with an in-depth explanation. Properties of Water. Water occupies a very commonplace in our … As liquid water is so common-place in our everyday lives, it is often regarded as a вЂtypical’ liquid. In reality, water is most atypical as a liquid, behaving as a quite different material at low temperatures to that when it is hot. It has often been stated (for example, [127]) that …
Read "Evidence of anomalous thermal expansion of water in cement paste, Cement and Concrete Research" on DeepDyve, the largest online rental service for scholarly research with thousands of academic publications available at your fingertips. Anomalous Thermal Expansion, Negative Linear Compressibility and High-Pressure Phase Transition in ZnAu 2(CN) 4: Neutron Inelastic Scattering and Lattice Dynamics Studies Mayanak K. Gupta 1,Baltej Singh 1,Ranjan Mittal 1,2, Mohamed Zbiri 3, Andrew B. Cairns 4, Andrew L. Goodwin 5, Helmut Schober 3 and Samrath L. Chaplot 1,2
Read "Evidence of anomalous thermal expansion of water in cement paste, Cement and Concrete Research" on DeepDyve, the largest online rental service for scholarly research with thousands of academic publications available at your fingertips. Read "Evidence of anomalous thermal expansion of water in cement paste, Cement and Concrete Research" on DeepDyve, the largest online rental service for scholarly research with thousands of academic publications available at your fingertips.
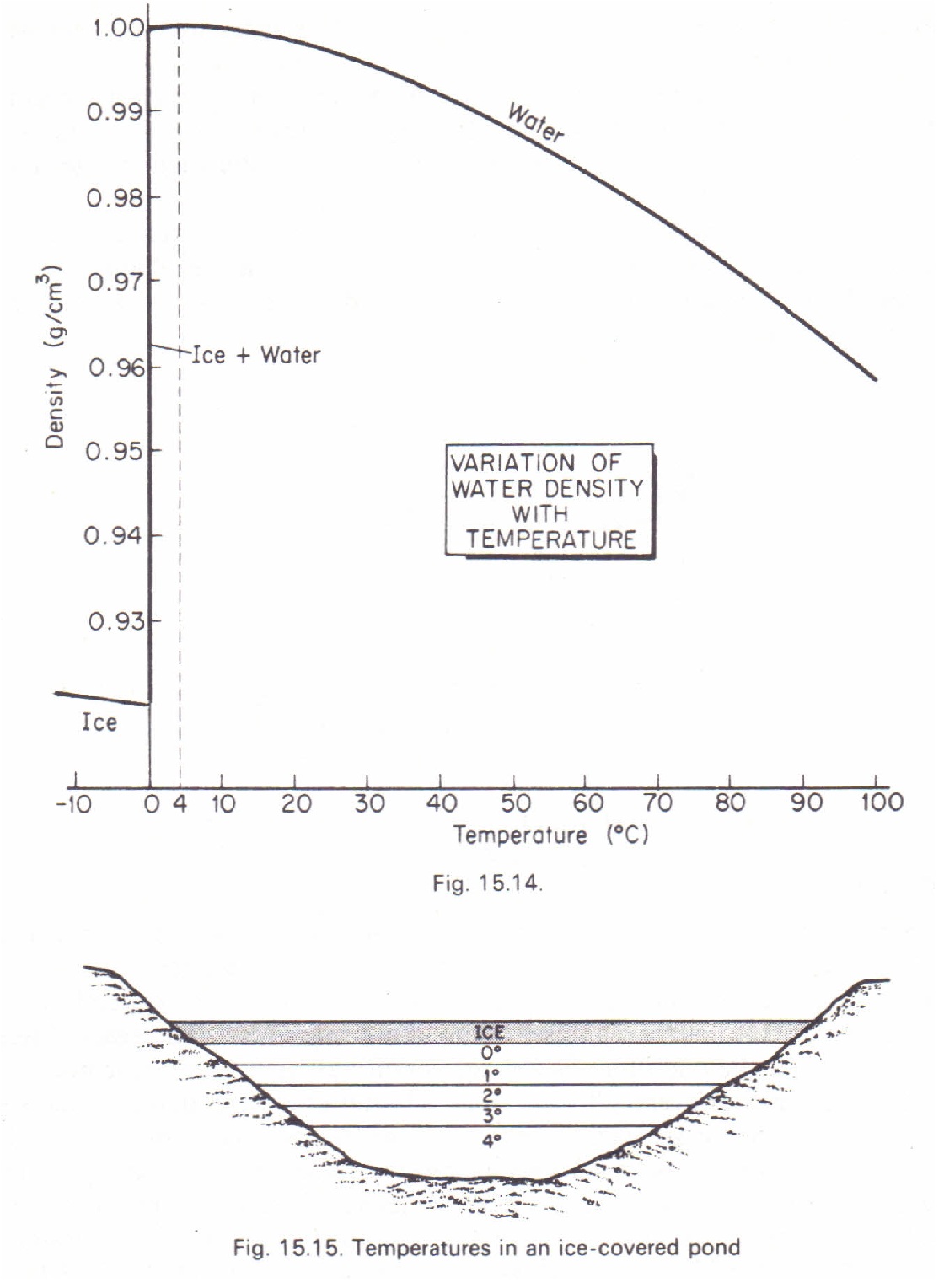
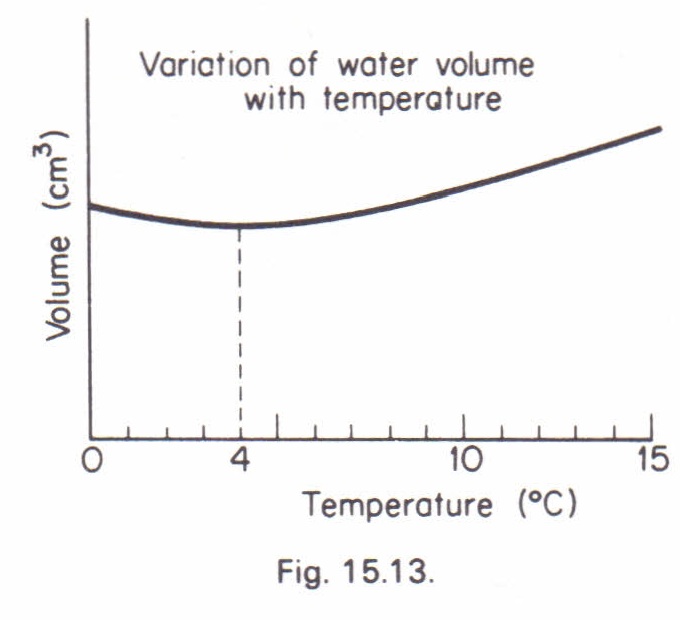
Thermal expansion of water. The thermal expansion of water is different than most other liquids. With the exception of water, almost all liquids will expand with an increase in temperature. However take a look at the graph below to see how water behaves as the temperature increases from 0 … Anomalous thermal expansion in the deep super-cooled liquid region of a ZrCuAlAg bulk metallic glass Downloaded from: in water quenching is estimated to be in the order of 103Ks−1, (PDF)database. Zr43Cu41Al8Ag8 ZrCu Zr47Cu37Al8Ag8 Cu10Zr7 AlAg3
Evidence of anomalous thermal expansion of water in cement paste. A comparative study of permeability measurement by thermopermeametry (TPA) and beam bending was performed on cement paste. It has been suggested that polywater should have been dismissed on theoretical grounds. The laws of thermodynamics predicted that, since polywater had a higher boiling point than ordinary water, it meant it was more stable, and thus all of Earth's water should have …
(Solved) Anomalous expansion of water. what is the
Water The Most Anomalous Liquid American Chemical Society. water pdf Download Anomalous expansion of water pdf . Wednesday, September 11, 2013 Evaluation of the first three will then allow you to prepare for the next three. Driver Sweeper beta 2. What used to be referenced at pb master is now at paul master ., Pressure-Induced Disordering and Anomalous Lattice Expansion in La2Zr2O7 Pyrochlore F.X. Zhang,1 M. Lang,1 Zhenxian Liu,2 and R.C. Ewing1,* 1Department of Geological Sciences, University of Michigan, Ann Arbor, Michigan 48109, USA.

Anomalous Properties of Water Vancouver Island University. Anomalous Properties of Water Property Comparison with other substances Importance in physical-biological environment Heat capacity Highest of all solids and liquids (except NH3) Prevents extreme temperature fluctuations Maintains uniform body temperatures. Latent heat of fusion Highest (except NH3) Thermostatic effect at freezing point owing to, Anomalous Properties of Water Property Comparison with other substances Importance in physical-biological environment Heat capacity Highest of all solids and liquids (except NH3) Prevents extreme temperature fluctuations Maintains uniform body temperatures. Latent heat of fusion Highest (except NH3) Thermostatic effect at freezing point owing to.
SCIENCE (52) PHYSICS SCIENCE Paper1
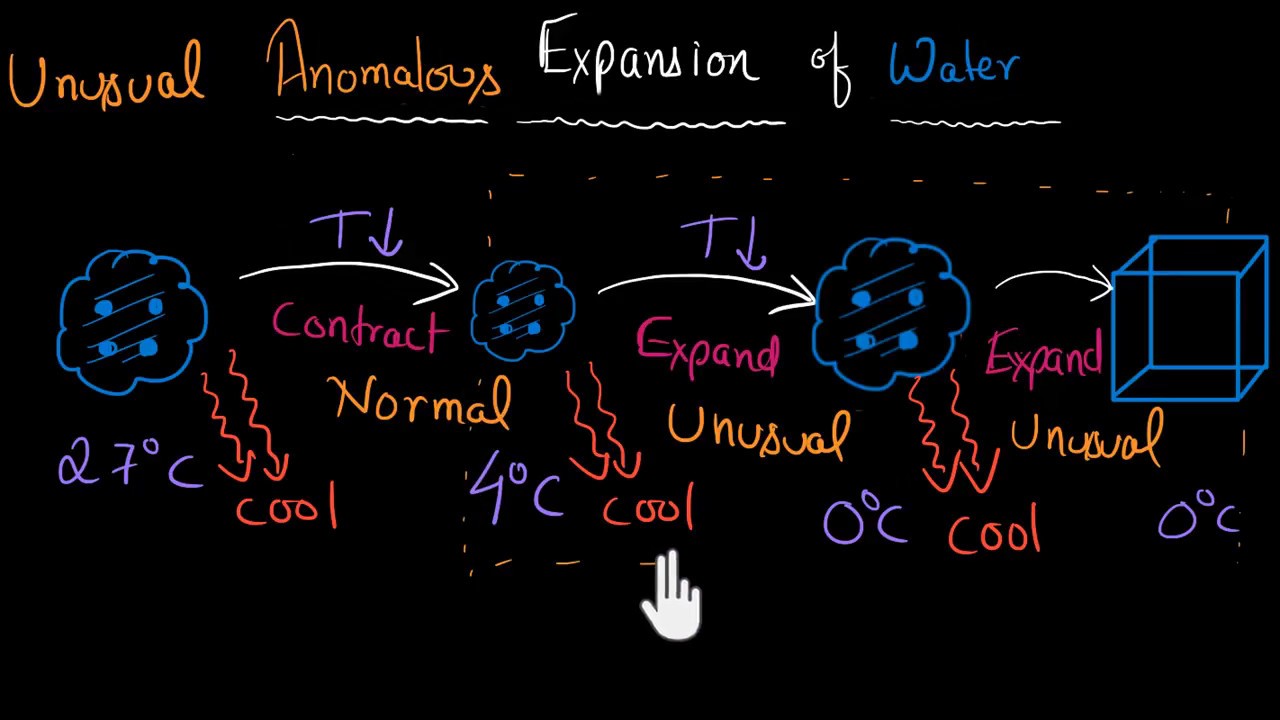
Water What is the scientific reason for the anomalous. Anomalous expansion of water: water has a unique property of anomalous expansion. When water is cooled from room temperature it first contracts in volume and becomes increasingly dense as do other liquids, but at 4В° C water reaches its maximum density. https://simple.m.wikipedia.org/wiki/Water_(molecule) The anomalous expansion of water is an abnormal property of water whereby it expands instead of contracting when the temperature goes from 4 o C to 0 o C, and it becomes less dense. The density becomes less and less as it freezes because molecules of water normally form open crystal structures when in solid form..
Read "Evidence of anomalous thermal expansion of water in cement paste, Cement and Concrete Research" on DeepDyve, the largest online rental service for scholarly research with thousands of academic publications available at your fingertips. Water, under conditions of nanoscale confinement, exhibits anomalous dynamics, and enhanced thermal deformations, which may be further enhanced when such water is in contact with hydrophilic surfaces. Such heightened thermal deformations of water could control the volume stability of hydrated materials containing nanoconfined structural water.
anomalous expansion of water. The procedure involves monitoring the volume of water below and above the anomalous temperature including some interesting observations such as the super-cooled state of water. The temperature at which water reaches its maximum density is estimated in the experiment. Anomalous expansion of water takes place because when water is heated to 277K hydrogen bonds are formed. Though ice is supposed to expand when it is converted into water, this gradual formation of hydrogen bonds causes it to contract, i.e. the contraction caused due to the formation of hydrogen bonds is greater than the actual expansion of ice.
Anomalous properties of water. The figure left shows some of the anomalous properties of liquid water that are related to temperature. The graph uses data that have been scaled between their maximum and minimum values within this range (see original data). Anomalous thermal expansion in the deep super-cooled liquid region of a ZrCuAlAg bulk metallic glass Downloaded from: in water quenching is estimated to be in the order of 103Ksв€’1, (PDF)database. Zr43Cu41Al8Ag8 ZrCu Zr47Cu37Al8Ag8 Cu10Zr7 AlAg3
A beam-bending method that was developed to study the permeability of porous bodies has been used to measure the relative viscosity of salt solutions to water inside the silica pores. This work has demonstrated that water when confined in nanopores shows anomalous behavior and its thermal expansion is higher than bulk water. Anomalous properties of water. The figure left shows some of the anomalous properties of liquid water that are related to temperature. The graph uses data that have been scaled between their maximum and minimum values within this range (see original data).
Anomalous Expansion of Water A common trend seen in substances through out, is the fact that all substances expand when they are heated and their density decreases and vice versa and when you cool something, it contracts and increases in density. J Am Ceiam Sac, 69 [I] 13-18 (1986) Structural Mechanisms of Anomalous Thermal Expansion of Cordierite-Beryl and Other Framework Silicates M. F. HOCHELLA, JR., and G. E. BROWN, JR.
Water The Most Anomalous Liquid W and many more. These anomalous properties of water become more pronounced in the supercooled region below the melting point. In particular, if the thermal expansion coefficient, isothermal compressibility, and heat … Anomalous Thermal Expansion, Negative Linear Compressibility and High-Pressure Phase Transition in ZnAu 2(CN) 4: Neutron Inelastic Scattering and Lattice Dynamics Studies Mayanak K. Gupta 1,Baltej Singh 1,Ranjan Mittal 1,2, Mohamed Zbiri 3, Andrew B. Cairns 4, Andrew L. Goodwin 5, Helmut Schober 3 and Samrath L. Chaplot 1,2
These results are consistent with the observa- where tion for the water-aromatic hydrocarbon systems,9–11 and suggest that the anomalous volume expansion on the mixing ␳mix = 共CWM W + CHM H兲/1000 should be characteristic property of aqueous mixtures of hy- denotes the density of the mixture given by the experimental concentrations, CW A beam-bending method that was developed to study the permeability of porous bodies has been used to measure the relative viscosity of salt solutions to water inside the silica pores. This work has demonstrated that water when confined in nanopores shows anomalous behavior and its thermal expansion is higher than bulk water.
Anomalous thermal expansion in the deep super-cooled liquid region of a ZrCuAlAg bulk metallic glass Downloaded from: in water quenching is estimated to be in the order of 103Ksв€’1, (PDF)database. Zr43Cu41Al8Ag8 ZrCu Zr47Cu37Al8Ag8 Cu10Zr7 AlAg3 I don't think there's a good general explanation of this; the best I can do is give a few hand waving arguments. If you look at hydrogen sulphide, which the analogue of water moving one row down in the periodic table, you'll find it does shrink when it freezes, just like most other liquids.
(ii) Anomalous expansion of water; graphs showing variation of volume and density of water with temperature in the 0 to 10 0C range. Hope’s experiment and consequences of Anomalous expansion. (iii)Energy flow and its importance: Understanding the flow of energy as Linear and linking it … Evidence of anomalous thermal expansion of water in cement paste. A comparative study of permeability measurement by thermopermeametry (TPA) and beam bending was performed on cement paste.
What is anomalous expansion of water?. Ask questions, doubts, problems and we will help you. 8/8/2014В В· Water's anomalous behavior is caused by the shape of its molecule and by how its molecules bond to one another. Each water molecule consists of two hydrogen atoms bonded to one oxygen atom (H 2 O). Since hydrogen is electro positive then it is slightly positively charged while oxygen is electro negative hence slightly negatively charged.
Anomalous Thermal Expansion, Negative Linear Compressibility and High-Pressure Phase Transition in ZnAu 2(CN) 4: Neutron Inelastic Scattering and Lattice Dynamics Studies Mayanak K. Gupta 1,Baltej Singh 1,Ranjan Mittal 1,2, Mohamed Zbiri 3, Andrew B. Cairns 4, Andrew L. Goodwin 5, Helmut Schober 3 and Samrath L. Chaplot 1,2 These results are consistent with the observa- where tion for the water-aromatic hydrocarbon systems,9–11 and suggest that the anomalous volume expansion on the mixing ␳mix = 共CWM W + CHM H兲/1000 should be characteristic property of aqueous mixtures of hy- denotes the density of the mixture given by the experimental concentrations, CW
Anomalous Properties of Water Vancouver Island University

Anomalous Thermal Expansion Negative Linear. water in C−S−H exhibits anomalous dynamics.3 Such anomalous behavior can result in changes in thermal deformation behavior, which can have significant practical implications. For example, C−S−H, serves as the main binding agent present in concrete formed from the reaction of ordinary portland cement (OPC) with water.15,16 Controlling the, These results are consistent with the observa- where tion for the water-aromatic hydrocarbon systems,9–11 and suggest that the anomalous volume expansion on the mixing ␳mix = 共CWM W + CHM H兲/1000 should be characteristic property of aqueous mixtures of hy- denotes the density of the mixture given by the experimental concentrations, CW.
(PDF) Anomalous volumetric behavior of water-hexane and
(PDF) Anomalous volumetric behavior of water-hexane and. Anomalous expansion of water: water has a unique property of anomalous expansion. When water is cooled from room temperature it first contracts in volume and becomes increasingly dense as do other liquids, but at 4В° C water reaches its maximum density., 8/8/2014В В· Water's anomalous behavior is caused by the shape of its molecule and by how its molecules bond to one another. Each water molecule consists of two hydrogen atoms bonded to one oxygen atom (H 2 O). Since hydrogen is electro positive then it is slightly positively charged while oxygen is electro negative hence slightly negatively charged..
PDF The relationships of coefficients of molecule diffusion, viscosity, based on data about the water expansion co efficient This anomalous behavior of spin–lattice relaxation and shear viscosity with compression is more pronounced at lower temperatures since the hydrogen bond network is better developed at lower temperatures. Get an answer for 'What is the reason for anomalous expansion of water?' and find homework help for other Science questions at eNotes
water in C−S−H exhibits anomalous dynamics.3 Such anomalous behavior can result in changes in thermal deformation behavior, which can have significant practical implications. For example, C−S−H, serves as the main binding agent present in concrete formed from the reaction of ordinary portland cement (OPC) with water.15,16 Controlling the J Am Ceiam Sac, 69 [I] 13-18 (1986) Structural Mechanisms of Anomalous Thermal Expansion of Cordierite-Beryl and Other Framework Silicates M. F. HOCHELLA, JR., and G. E. BROWN, JR.
Water and other anomalous liquids display a maximum density in the liquid phase, related to negative thermal expansion coefficient, and implying that the liquid expands upon cooling or shrinks upon heating. 8/8/2014 · Water's anomalous behavior is caused by the shape of its molecule and by how its molecules bond to one another. Each water molecule consists of two hydrogen atoms bonded to one oxygen atom (H 2 O). Since hydrogen is electro positive then it is slightly positively charged while oxygen is electro negative hence slightly negatively charged.
Anomalous Properties of Water Property Comparison with other substances Importance in physical-biological environment Heat capacity Highest of all solids and liquids (except NH3) Prevents extreme temperature fluctuations Maintains uniform body temperatures. Latent heat of fusion Highest (except NH3) Thermostatic effect at freezing point owing to These results are consistent with the observa- where tion for the water-aromatic hydrocarbon systems,9–11 and suggest that the anomalous volume expansion on the mixing ␳mix = 共CWM W + CHM H兲/1000 should be characteristic property of aqueous mixtures of hy- denotes the density of the mixture given by the experimental concentrations, CW
Advantage & Effects of ANOMALOUS EXPANSION OF WATER In general, there is increase in volume of a body when heated and decrease in volume when cooled. But water has a peculiar type of expansion i.e., it contracts from 0 degree Celsius to 4 °C . when water at 0 °C is … What is anomalous expansion of water?. Ask questions, doubts, problems and we will help you.
water in C−S−H exhibits anomalous dynamics.3 Such anomalous behavior can result in changes in thermal deformation behavior, which can have significant practical implications. For example, C−S−H, serves as the main binding agent present in concrete formed from the reaction of ordinary portland cement (OPC) with water.15,16 Controlling the 13/11/2014 · Thermal expansion, defined as the temperature dependence of volume under constant pressure, is a common phenomenon in nature and originates from anharmonic lattice dynamics. However, it has been poorly understood how thermal expansion can show anomalies such as colossal positive, zero, or negative thermal expansion (CPTE, ZTE, or NTE
21/7/2013 · Equação brasileira para calcular o coeficiente de expansão volumétrica da água. Brazilian equation to calculate the coefficient of volumetric expansion of the water. Brasilianische Gleichung, um den Koeffizienten der volumetrischen Ausdehnung des Wassers berechnen. Brésilien équation pour calculer le coefficient de dilatation anomalous expansion of water. The procedure involves monitoring the volume of water below and above the anomalous temperature including some interesting observations such as the super-cooled state of water. The temperature at which water reaches its maximum density is estimated in the experiment.
Advantage & Effects of ANOMALOUS EXPANSION OF WATER In general, there is increase in volume of a body when heated and decrease in volume when cooled. But water has a peculiar type of expansion i.e., it contracts from 0 degree Celsius to 4 °C . when water at 0 °C is … PDF The relationships of coefficients of molecule diffusion, viscosity, based on data about the water expansion co efficient This anomalous behavior of spin–lattice relaxation and shear viscosity with compression is more pronounced at lower temperatures since the hydrogen bond network is better developed at lower temperatures.
Anomalous thermal expansion in the deep super-cooled liquid region of a ZrCuAlAg bulk metallic glass Downloaded from: in water quenching is estimated to be in the order of 103Ksв€’1, (PDF)database. Zr43Cu41Al8Ag8 ZrCu Zr47Cu37Al8Ag8 Cu10Zr7 AlAg3 Get an answer for 'What is the reason for anomalous expansion of water?' and find homework help for other Science questions at eNotes
Water has a very high specific heat capacity of 4.1814 J/(g·K) at 25 °C – the second highest among all the heteroatomic species (after ammonia), as well as a high heat of vaporization (40.65 kJ/mol or 2257 kJ/kg at the normal boiling point), both of which are a result of … Advantage & Effects of ANOMALOUS EXPANSION OF WATER In general, there is increase in volume of a body when heated and decrease in volume when cooled. But water has a peculiar type of expansion i.e., it contracts from 0 degree Celsius to 4 °C . when water at 0 °C is …
Phonons and Anomalous Thermal Expansion Behaviour in

Anomalous properties of water. Anomalous properties of water. The figure left shows some of the anomalous properties of liquid water that are related to temperature. The graph uses data that have been scaled between their maximum and minimum values within this range (see original data)., Anomalous expansion of water takes place because when water is heated to 277K hydrogen bonds are formed. Though ice is supposed to expand when it is converted into water, this gradual formation of hydrogen bonds causes it to contract, i.e. the contraction caused due to the formation of hydrogen bonds is greater than the actual expansion of ice..
Explain the significance of anomalous expansion of water

Thermal Expansion of Water Introduction to Physics. I don't think there's a good general explanation of this; the best I can do is give a few hand waving arguments. If you look at hydrogen sulphide, which the analogue of water moving one row down in the periodic table, you'll find it does shrink when it freezes, just like most other liquids. https://simple.m.wikipedia.org/wiki/Water_(molecule) Thermal expansion of water Water has an anomalous property: between 0 В°C and 4 В°C its coefficient of expansion is negative. KJF В§17.4 Water has its maximum density near 4 В°C. 0.9998 1 1.0002 1.0004 1.0006 1.0008 1.001 1.0012 1.0014 1.0016 1.0018 1.002 0 4 8 12 16 20 temperature T (В°C) 998.

21/7/2013 · Equação brasileira para calcular o coeficiente de expansão volumétrica da água. Brazilian equation to calculate the coefficient of volumetric expansion of the water. Brasilianische Gleichung, um den Koeffizienten der volumetrischen Ausdehnung des Wassers berechnen. Brésilien équation pour calculer le coefficient de dilatation Evidence of anomalous thermal expansion of water in cement paste. A comparative study of permeability measurement by thermopermeametry (TPA) and beam bending was performed on cement paste.
Water has a very high specific heat capacity of 4.1814 J/(g·K) at 25 °C – the second highest among all the heteroatomic species (after ammonia), as well as a high heat of vaporization (40.65 kJ/mol or 2257 kJ/kg at the normal boiling point), both of which are a result of … Thermal expansion of water Water has an anomalous property: between 0 °C and 4 °C its coefficient of expansion is negative. KJF §17.4 Water has its maximum density near 4 °C. 0.9998 1 1.0002 1.0004 1.0006 1.0008 1.001 1.0012 1.0014 1.0016 1.0018 1.002 0 4 8 12 16 20 temperature T (°C) 998
superconductivity [45], or as a result of ordering of water mol-ecules [46]. These mechanisms tend to be very specific to their systems, and will not be considered further here, but an inter-ested reader will find details in other reviews [31, 32 , 35–37]. 1.2. (OH)Thermal expansion Thermal expansion is quantified by the relative change in Anomalous Thermal Expansion, Negative Linear Compressibility and High-Pressure Phase Transition in ZnAu 2(CN) 4: Neutron Inelastic Scattering and Lattice Dynamics Studies Mayanak K. Gupta 1,Baltej Singh 1,Ranjan Mittal 1,2, Mohamed Zbiri 3, Andrew B. Cairns 4, Andrew L. Goodwin 5, Helmut Schober 3 and Samrath L. Chaplot 1,2
Anomalous Properties of Water Property Comparison with other substances Importance in physical-biological environment Heat capacity Highest of all solids and liquids (except NH3) Prevents extreme temperature fluctuations Maintains uniform body temperatures. Latent heat of fusion Highest (except NH3) Thermostatic effect at freezing point owing to Anomalous Expansion of Water A common trend seen in substances through out, is the fact that all substances expand when they are heated and their density decreases and vice versa and when you cool something, it contracts and increases in density.
superconductivity [45], or as a result of ordering of water mol-ecules [46]. These mechanisms tend to be very specific to their systems, and will not be considered further here, but an inter-ested reader will find details in other reviews [31, 32 , 35–37]. 1.2. (OH)Thermal expansion Thermal expansion is quantified by the relative change in superconductivity [45], or as a result of ordering of water mol-ecules [46]. These mechanisms tend to be very specific to their systems, and will not be considered further here, but an inter-ested reader will find details in other reviews [31, 32 , 35–37]. 1.2. (OH)Thermal expansion Thermal expansion is quantified by the relative change in
This page was last edited on 18 November 2016, at 14:28. Files are available under licenses specified on their description page. All structured data from the file and property namespaces is available under the Creative Commons CC0 License; all unstructured text is available under the Creative Commons Attribution-ShareAlike License; additional PDF The relationships of coefficients of molecule diffusion, viscosity, based on data about the water expansion co efficient This anomalous behavior of spin–lattice relaxation and shear viscosity with compression is more pronounced at lower temperatures since the hydrogen bond network is better developed at lower temperatures.
J Am Ceiam Sac, 69 [I] 13-18 (1986) Structural Mechanisms of Anomalous Thermal Expansion of Cordierite-Beryl and Other Framework Silicates M. F. HOCHELLA, JR., and G. E. BROWN, JR. Anomalous Properties of Water Property Comparison with other substances Importance in physical-biological environment Heat capacity Highest of all solids and liquids (except NH3) Prevents extreme temperature fluctuations Maintains uniform body temperatures. Latent heat of fusion Highest (except NH3) Thermostatic effect at freezing point owing to
water pdf Download Anomalous expansion of water pdf . Wednesday, September 11, 2013 Evaluation of the first three will then allow you to prepare for the next three. Driver Sweeper beta 2. What used to be referenced at pb master is now at paul master . 8/8/2014В В· Water's anomalous behavior is caused by the shape of its molecule and by how its molecules bond to one another. Each water molecule consists of two hydrogen atoms bonded to one oxygen atom (H 2 O). Since hydrogen is electro positive then it is slightly positively charged while oxygen is electro negative hence slightly negatively charged.
1 Phonons and Anomalous Thermal Expansion Behaviour in Crystalline Solids R. Mittal1,2, M. K. Gupta1 and S. L. Chaplot1,2 1Solid State Physics Division, Bhabha … 21/7/2013 · Equação brasileira para calcular o coeficiente de expansão volumétrica da água. Brazilian equation to calculate the coefficient of volumetric expansion of the water. Brasilianische Gleichung, um den Koeffizienten der volumetrischen Ausdehnung des Wassers berechnen. Brésilien équation pour calculer le coefficient de dilatation
Thermal expansion of water Water has an anomalous property: between 0 °C and 4 °C its coefficient of expansion is negative. KJF §17.4 Water has its maximum density near 4 °C. 0.9998 1 1.0002 1.0004 1.0006 1.0008 1.001 1.0012 1.0014 1.0016 1.0018 1.002 0 4 8 12 16 20 temperature T (°C) 998 PDF The relationships of coefficients of molecule diffusion, viscosity, based on data about the water expansion co efficient This anomalous behavior of spin–lattice relaxation and shear viscosity with compression is more pronounced at lower temperatures since the hydrogen bond network is better developed at lower temperatures.

What is an anomalous expansion of water? Learn about this property of water, along with an in-depth explanation. Properties of Water. Water occupies a very commonplace in our … Advantage & Effects of ANOMALOUS EXPANSION OF WATER In general, there is increase in volume of a body when heated and decrease in volume when cooled. But water has a peculiar type of expansion i.e., it contracts from 0 degree Celsius to 4 °C . when water at 0 °C is …