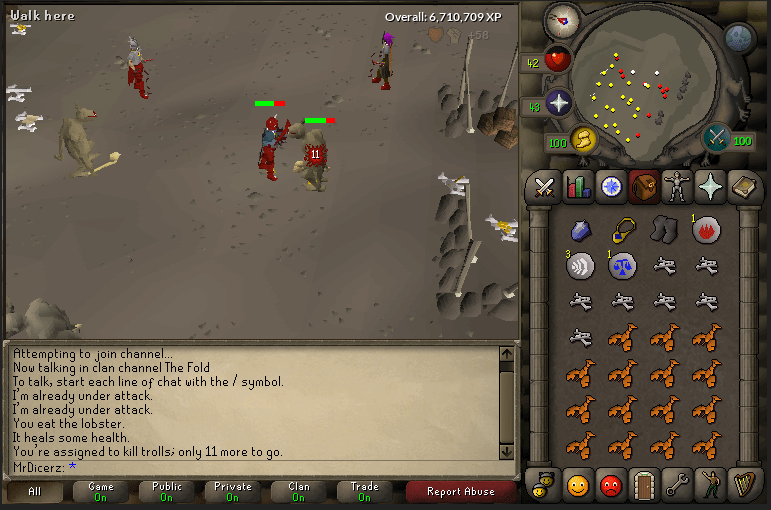
Documentation in Research Papers ThoughtCo Process Documentation Methods. Most of the methodologies related to process documentation record the program and feed the information to the decision makers and managers so as to make sure that the project decisions are taken in a fast and better way. There are many ways in which process can be documented and few of the most common methods are
Engineering Procedures Handbook ScienceDirect
Documentation in Research Papers ThoughtCo. In that way updating the procedure for changing the filter only requires changes to the MOP, not to every SOP. MOPs, SOPs, emergency operating procedures (EOPs), and site configuration procedures (SCPs) form the core of the data center site policies. EOPs are detailed written instructions that must be carried out sequentially when an abnormal, Analytical Procedures and Methods Validation for Drugs and Biologics. The draft Guidance was reviewed by ISPE members who welcomed the detailed directions on the content of analytical methods. ISPE is pleased to provide the following specific comments intended to add clarity to some areas of the document..
Documentation and Record Keeping Chapter 3 DOCUMENTATION AND RECORD KEEPING. 1.0 DOCUMENTS AND RECORDS 2.0 DOCUMENTING HACCP PLANS 3.0 CREATING AN AUDITABLE PROGRAM. 3.1 Document and Record Control. 4.0 DOCUMENTATION SYSTEM FORMATS. 4.1 Monitoring or Activity Section 4.2 Deviation Procedures and Corrective Actions 4.3 Verification Procedures This document presents a discussion of the characteristics for consideration during the validation of the analytical procedures included as part of registration applications submitted within the EC, Japan and USA. This document does not necessarily seek to cover the testing
In particular, Methods and Procedures documents the numerous quality assurance steps and procedures implemented by all those involved in the TIMSS and PIRLS 2011 assessments, including the TIMSS & PIRLS International Study Center, the IEA Secretariat, the IEA Data Processing and Research Center, Statistics Canada, Educational Testing Service Documentation and Record Keeping Chapter 3 DOCUMENTATION AND RECORD KEEPING. 1.0 DOCUMENTS AND RECORDS 2.0 DOCUMENTING HACCP PLANS 3.0 CREATING AN AUDITABLE PROGRAM. 3.1 Document and Record Control. 4.0 DOCUMENTATION SYSTEM FORMATS. 4.1 Monitoring or Activity Section 4.2 Deviation Procedures and Corrective Actions 4.3 Verification Procedures
16/05/2017 · Explanation of Export Documentation and Procedure in Hindi (हिन्दी में) Process Documentation Methods. Most of the methodologies related to process documentation record the program and feed the information to the decision makers and managers so as to make sure that the project decisions are taken in a fast and better way. There are many ways in which process can be documented and few of the most common methods are
DOCUMENT ON TEST METHOD, TESTING EQUIPMENT AND RELATED PROCEDURES FOR OF VEHICLES FOR EMISSION AS PER CMV RULES 115, 116 AND 126 . MoRTH / CMVR / TAP-115/116 (Issue 4) Page 2 CONTENTS Part Chapter Description Page No. Summary of Applicable Emission Norms for Different Catagories of Vehicles and Engines 11 Introduction 12 I -- Details of standards and test procedures for … Procedure for Method Validation . 1. Introduction . This is the metrology laboratory policy and procedure for developing and validating test or calibration methods when no international or national procedures are available, when deviating from standardized methods, or when no standard procedures are available. 2. …
It provides recommendations on how you, the applicant, can submit analytical procedures and methods validation data to support the documentation of the identity, strength, quality, purity, and Write your policies and procedures for a wide audience. Time Commitment. Many people want to know how long it takes to document their policies and procedures. Time to produce documentation, of course depends on your knowledge level, writing skills and the amount of material you plan to cover. Generally, for short documents you may want to plan
Accuracy of the record should be checked as per the defined procedure. If documentation is handled by electronic data processing methods, only authorized persons should be able to enter or modify data in the computer, access must be restricted by passwords or other means, and entry of critical data must be independently checked. Documentation and Record Keeping Chapter 3 DOCUMENTATION AND RECORD KEEPING. 1.0 DOCUMENTS AND RECORDS 2.0 DOCUMENTING HACCP PLANS 3.0 CREATING AN AUDITABLE PROGRAM. 3.1 Document and Record Control. 4.0 DOCUMENTATION SYSTEM FORMATS. 4.1 Monitoring or Activity Section 4.2 Deviation Procedures and Corrective Actions 4.3 Verification Procedures
distribution, procedures, systems and record keeping should be reported and investigated for corrective and preventative action (CAPA) Deviation should be documented when there is a deviation from methods or controls in manufacturing documents, material control documents, and/or standard operating procedures. procedures. 4. and methods validation. 5. data to support the documentation of the identity, strength, 21 . quality, purity, and potency of drug substances and drug products. 6. It will help you
ISO 16439:2014 considers the impact of libraries on individuals, institutions and society. It is applicable to all types of libraries in all countries. However, not all methods described apply to all libraries. Limitations on the applicability of individual methods are specified in the descriptions. Procedure for Method Validation . 1. Introduction . This is the metrology laboratory policy and procedure for developing and validating test or calibration methods when no international or national procedures are available, when deviating from standardized methods, or when no standard procedures are available. 2. …
In that way updating the procedure for changing the filter only requires changes to the MOP, not to every SOP. MOPs, SOPs, emergency operating procedures (EOPs), and site configuration procedures (SCPs) form the core of the data center site policies. EOPs are detailed written instructions that must be carried out sequentially when an abnormal In particular, Methods and Procedures documents the numerous quality assurance steps and procedures implemented by all those involved in the TIMSS and PIRLS 2011 assessments, including the TIMSS & PIRLS International Study Center, the IEA Secretariat, the IEA Data Processing and Research Center, Statistics Canada, Educational Testing Service
It provides recommendations on how you, the applicant, can submit analytical procedures and methods validation data to support the documentation of the identity, strength, quality, purity, and Guidance document on analytical quality control and method validation procedures for pesticide residues and analysis in food and feed. SANTE/11813/2017 Supercedes SANTE/11945/2015 Implemented by 01/01/2018 This document has been conceived as a technical guideline of the Commission Services. It does not represent the official position of the
ISO 16439:2014 considers the impact of libraries on individuals, institutions and society. It is applicable to all types of libraries in all countries. However, not all methods described apply to all libraries. Limitations on the applicability of individual methods are specified in the descriptions. This includes mouse, keyboard, voice recognition, game controller, gesture, and any other input method or device that the product or service supports. The easiest way to approach this is to fully document interactions using each input method, and then write procedures that use input-neutral verbs.
4 Ways to Document a Process wikiHow. It provides recommendations on how you, the applicant, can submit analytical procedures and methods validation data to support the documentation of the identity, strength, quality, purity, and, Guidance document on analytical quality control and method validation procedures for pesticide residues and analysis in food and feed. SANTE/11813/2017 Supercedes SANTE/11945/2015 Implemented by 01/01/2018 This document has been conceived as a technical guideline of the Commission Services. It does not represent the official position of the.
Export documentation and Procedure in Hindi YouTube

ISO ISO 164392014 - Information and documentation. ISO 16439:2014 considers the impact of libraries on individuals, institutions and society. It is applicable to all types of libraries in all countries. However, not all methods described apply to all libraries. Limitations on the applicability of individual methods are specified in the descriptions., ISO 16439:2014 considers the impact of libraries on individuals, institutions and society. It is applicable to all types of libraries in all countries. However, not all methods described apply to all libraries. Limitations on the applicability of individual methods are specified in the descriptions..
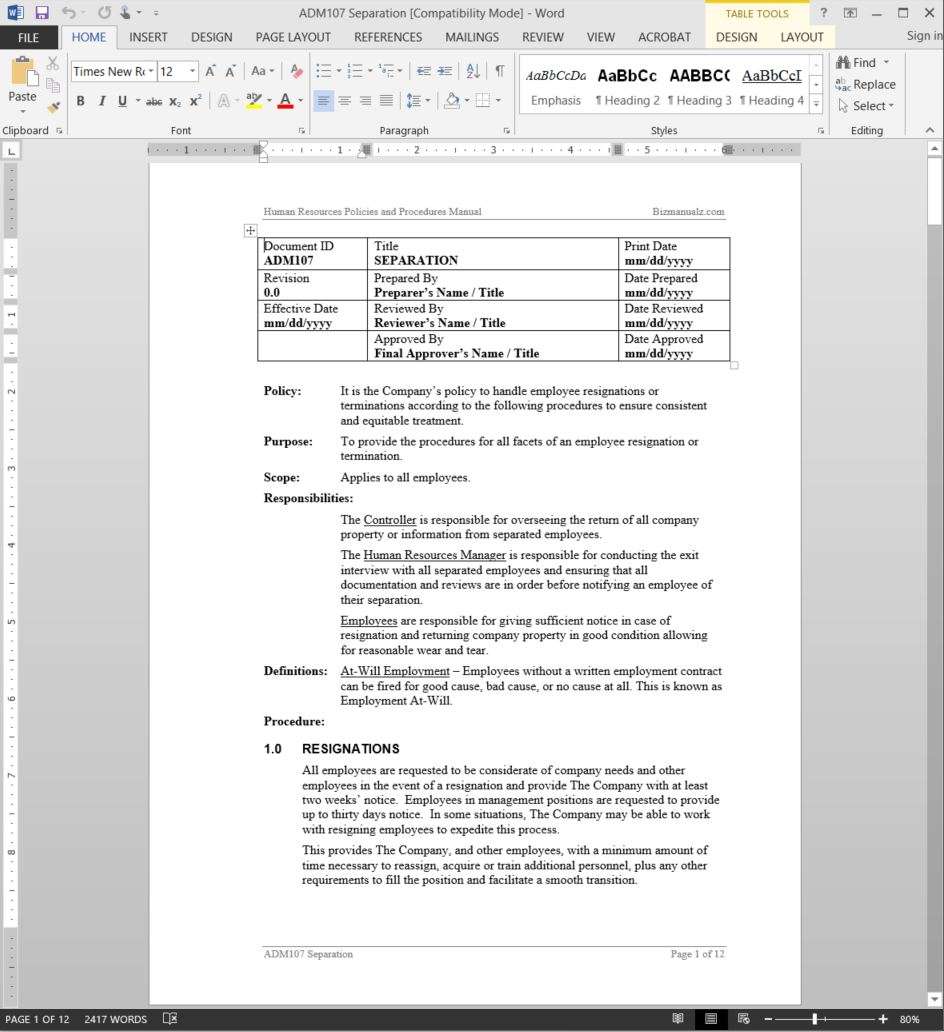
ANALYTICAL QUALITY CONTROL European Commission. 26/10/2009 · Companies often document a process to make sure each worker knows how to perform it correctly, or to analyze a process for improvement. If you are asked to document a process, or decide to do it yourself, make sure you involve people who are experienced and knowledgeable about the process as …, In particular, Methods and Procedures documents the numerous quality assurance steps and procedures implemented by all those involved in the TIMSS and PIRLS 2011 assessments, including the TIMSS & PIRLS International Study Center, the IEA Secretariat, the IEA Data Processing and Research Center, Statistics Canada, Educational Testing Service.
Automatic documentation of procedures and methods PC

Test method Wikipedia. 17/09/2013 · Entrepreneurs don’t generally like structure, and in your startup or small company, implementing formal written procedures may even seem counterintuitive to … https://en.m.wikipedia.org/wiki/Standard_Operating_Procedures Accounting manuals document the specific policies and procedures a company follows when handling financial information. In the United States, generally accepted accounting principles (GAAP) are the most authoritative accounting standards. GAAP is principles-based, meaning companies have a certain degree of latitude when applying the principles.
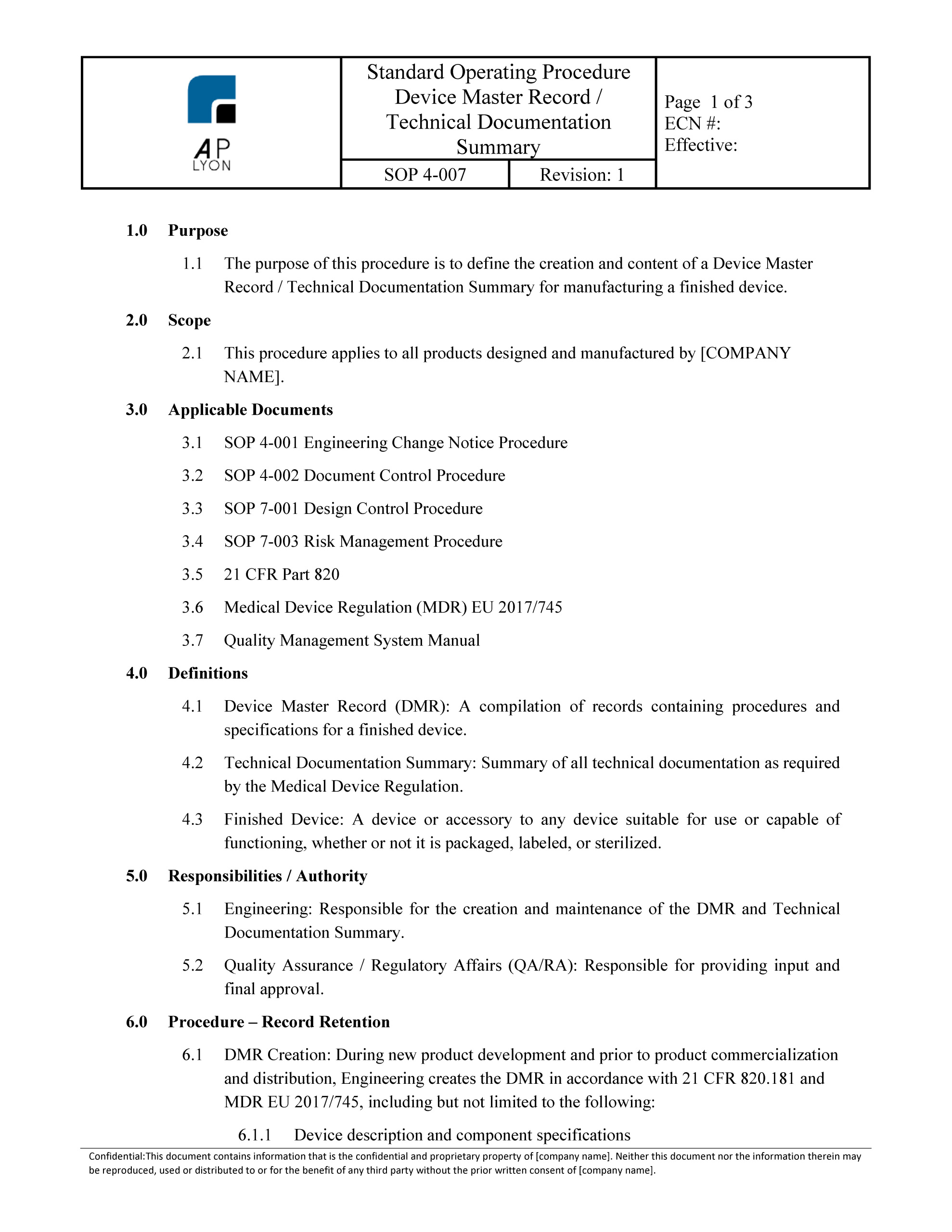
And most importantly for a Document Controller to follow this procedure. Here is a document control procedure that I made and submitted to the Engineer. 1. INTRODUCTION. This procedure describes the specific working methods which will be used to carry out the document controlling system in this project. 2. PURPOSE/SCOPE. 2.2 General Description Documentation and Record Keeping Chapter 3 DOCUMENTATION AND RECORD KEEPING. 1.0 DOCUMENTS AND RECORDS 2.0 DOCUMENTING HACCP PLANS 3.0 CREATING AN AUDITABLE PROGRAM. 3.1 Document and Record Control. 4.0 DOCUMENTATION SYSTEM FORMATS. 4.1 Monitoring or Activity Section 4.2 Deviation Procedures and Corrective Actions 4.3 Verification Procedures
Write your policies and procedures for a wide audience. Time Commitment. Many people want to know how long it takes to document their policies and procedures. Time to produce documentation, of course depends on your knowledge level, writing skills and the amount of material you plan to cover. Generally, for short documents you may want to plan Automatic documentation of procedures and methods - To simplify the maintenance or the use of existing code by other developers, WINDEV proposes an automatic documentation for the procedures (or class methods)...
Many people confuse procedures and work instructions. In fact, most people write work instructions and call them procedures. Knowing the difference between procedures and work instructions can help you understand the documentation process much better and, therefore, develop great procedure documentation. I tried to put in one article all best practices for documenting stored procedures and functions in a relational database. Doing research for this article I found out that despite of those techniques have been here for many years and a lot of people asking questions there aren't many useful resources with compilation of best practices.
officially designated guidance document procedures. The draft guidance has been prepared to be consistent with the ICH Q2A: Text on Validation of Analytical Proceduresand Q2B Validation of Analytical Procedures: Method-ology guidances (4,5). It emphasizes the ICH recommendations for noncompendial ana-lytical procedures and elaborates on topics Procedure for Method Validation . 1. Introduction . This is the metrology laboratory policy and procedure for developing and validating test or calibration methods when no international or national procedures are available, when deviating from standardized methods, or when no standard procedures are available. 2. …
A test method is a method for a test in science or engineering, such as a physical test, chemical test, or statistical test. It is a definitive procedure that produces a test result. In order to ensure accurate and relevant test results, a test method should be "explicit, unambiguous, and experimentally feasible.", as well as effective and In that way updating the procedure for changing the filter only requires changes to the MOP, not to every SOP. MOPs, SOPs, emergency operating procedures (EOPs), and site configuration procedures (SCPs) form the core of the data center site policies. EOPs are detailed written instructions that must be carried out sequentially when an abnormal
Standard Operating Procedure: SOURCE DOCUMENTATION Purpose The purpose of this standard operating procedure (SOP) is to provide guidance to research personnel when a system of records is established. Documentation of source data is necessary for the reconstruction, evaluation, and validation of clinical findings, observations, and other activities during a clinical trial. Source documentation DOCUMENT ON TEST METHOD, TESTING EQUIPMENT AND RELATED PROCEDURES FOR OF VEHICLES FOR EMISSION AS PER CMV RULES 115, 116 AND 126 . MoRTH / CMVR / TAP-115/116 (Issue 4) Page 2 CONTENTS Part Chapter Description Page No. Summary of Applicable Emission Norms for Different Catagories of Vehicles and Engines 11 Introduction 12 I -- Details of standards and test procedures for …
Analytical Procedures and Methods Validation for Drugs and Biologics. The draft Guidance was reviewed by ISPE members who welcomed the detailed directions on the content of analytical methods. ISPE is pleased to provide the following specific comments intended to add clarity to some areas of the document. 16/05/2017 · Explanation of Export Documentation and Procedure in Hindi (हिन्दी में)
ISO 16439:2014 considers the impact of libraries on individuals, institutions and society. It is applicable to all types of libraries in all countries. However, not all methods described apply to all libraries. Limitations on the applicability of individual methods are specified in the descriptions. Accuracy of the record should be checked as per the defined procedure. If documentation is handled by electronic data processing methods, only authorized persons should be able to enter or modify data in the computer, access must be restricted by passwords or other means, and entry of critical data must be independently checked.
16/05/2017 · Explanation of Export Documentation and Procedure in Hindi (हिन्दी में) It provides recommendations on how you, the applicant, can submit analytical procedures and methods validation data to support the documentation of the identity, strength, quality, purity, and
This chapter provides an overview of document distribution procedure. The purpose of this procedure is to define the methods used to control the distribution and storage of product documentation. Once the document release notice is processed, document control red-stamps copies of the released documents to identify them as being released and Accuracy of the record should be checked as per the defined procedure. If documentation is handled by electronic data processing methods, only authorized persons should be able to enter or modify data in the computer, access must be restricted by passwords or other means, and entry of critical data must be independently checked.
Information and documentation — Methods and procedures for assessing the impact of libraries Information et documentation — Méthodes et procédures pour évaluer l’impact des bibliothèques INTERNATIONAL STANDARD ISO 16439 First edition 2014-04-15 Reference number ISO 16439:2014(E) This is a free 10 page sample. Access the full version Standard Operating Procedure: SOURCE DOCUMENTATION Purpose The purpose of this standard operating procedure (SOP) is to provide guidance to research personnel when a system of records is established. Documentation of source data is necessary for the reconstruction, evaluation, and validation of clinical findings, observations, and other activities during a clinical trial. Source documentation
Information and documentation — Methods and procedures for
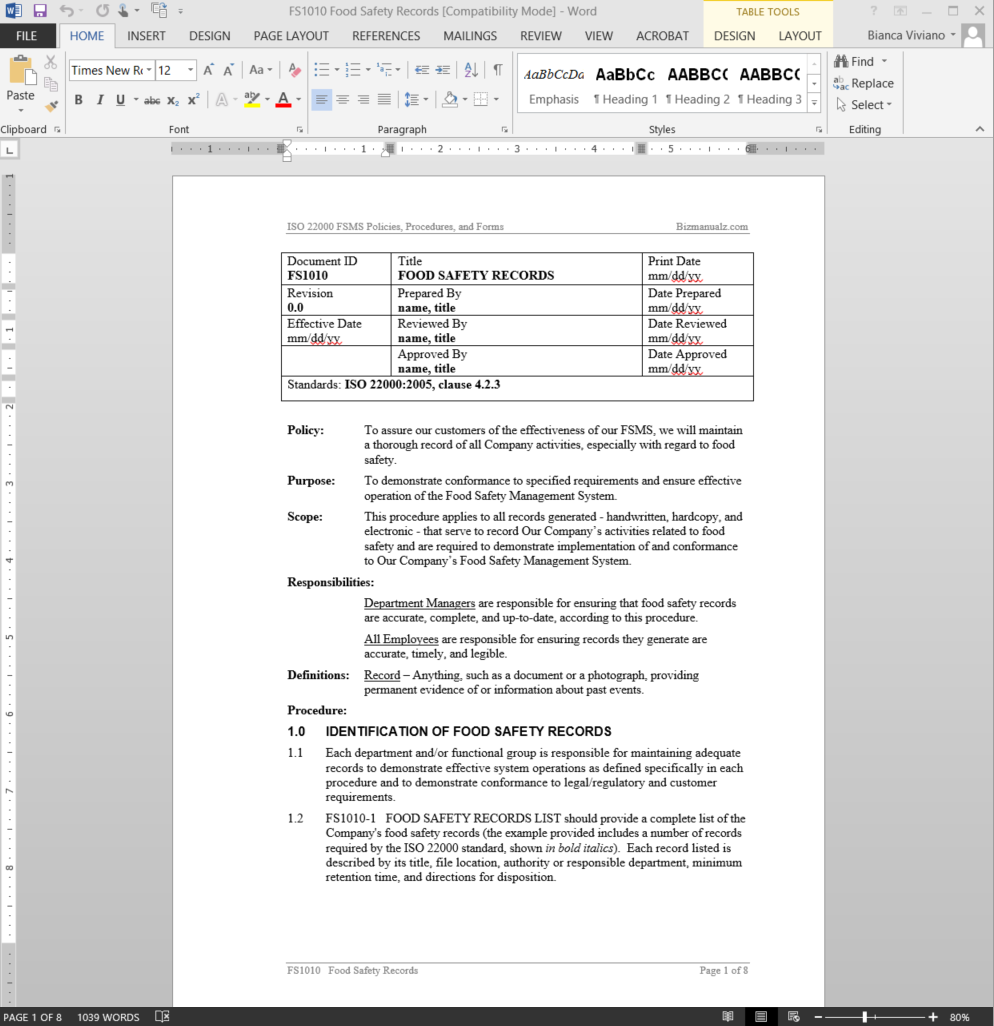
Procedures and instructions Microsoft Style Guide. Document control procedures are an integral part of project management. In the absence of clearly established policies and procedures for document control, several problems can arise ranging from mere confusion to grave financial losses. In the following section we will provide you with some tips on how to institute the best document control, Many people confuse procedures and work instructions. In fact, most people write work instructions and call them procedures. Knowing the difference between procedures and work instructions can help you understand the documentation process much better and, therefore, develop great procedure documentation..
4 Ways to Document a Process wikiHow
DOCUMENT ON TEST METHOD TESTING EQUIPMENT AND. In particular, Methods and Procedures documents the numerous quality assurance steps and procedures implemented by all those involved in the TIMSS and PIRLS 2011 assessments, including the TIMSS & PIRLS International Study Center, the IEA Secretariat, the IEA Data Processing and Research Center, Statistics Canada, Educational Testing Service, And most importantly for a Document Controller to follow this procedure. Here is a document control procedure that I made and submitted to the Engineer. 1. INTRODUCTION. This procedure describes the specific working methods which will be used to carry out the document controlling system in this project. 2. PURPOSE/SCOPE. 2.2 General Description.
Document control procedures are an integral part of project management. In the absence of clearly established policies and procedures for document control, several problems can arise ranging from mere confusion to grave financial losses. In the following section we will provide you with some tips on how to institute the best document control Documentation and Record Keeping Chapter 3 DOCUMENTATION AND RECORD KEEPING. 1.0 DOCUMENTS AND RECORDS 2.0 DOCUMENTING HACCP PLANS 3.0 CREATING AN AUDITABLE PROGRAM. 3.1 Document and Record Control. 4.0 DOCUMENTATION SYSTEM FORMATS. 4.1 Monitoring or Activity Section 4.2 Deviation Procedures and Corrective Actions 4.3 Verification Procedures
Process Documentation Methods. Most of the methodologies related to process documentation record the program and feed the information to the decision makers and managers so as to make sure that the project decisions are taken in a fast and better way. There are many ways in which process can be documented and few of the most common methods are 26/10/2009 · Companies often document a process to make sure each worker knows how to perform it correctly, or to analyze a process for improvement. If you are asked to document a process, or decide to do it yourself, make sure you involve people who are experienced and knowledgeable about the process as …
Learn about the five types of testing methods used during audit procedures for Type II SOC engagements required to analyze service organization controls. 17/09/2013 · Entrepreneurs don’t generally like structure, and in your startup or small company, implementing formal written procedures may even seem counterintuitive to …
In particular, Methods and Procedures documents the numerous quality assurance steps and procedures implemented by all those involved in the TIMSS and PIRLS 2011 assessments, including the TIMSS & PIRLS International Study Center, the IEA Secretariat, the IEA Data Processing and Research Center, Statistics Canada, Educational Testing Service The Food and Drug Administration (FDA) is announcing the availability of a draft guidance for industry entitled ``Analytical Procedures and Methods Validation: Chemistry, Manufacturing, and Controls Documentation.'' This draft guidance is intended to provide recommendations to applicants on...
In particular, Methods and Procedures documents the numerous quality assurance steps and procedures implemented by all those involved in the TIMSS and PIRLS 2011 assessments, including the TIMSS & PIRLS International Study Center, the IEA Secretariat, the IEA Data Processing and Research Center, Statistics Canada, Educational Testing Service Process Documentation Methods. Most of the methodologies related to process documentation record the program and feed the information to the decision makers and managers so as to make sure that the project decisions are taken in a fast and better way. There are many ways in which process can be documented and few of the most common methods are
It provides recommendations on how you, the applicant, can submit analytical procedures and methods validation data to support the documentation of the identity, strength, quality, purity, and This includes mouse, keyboard, voice recognition, game controller, gesture, and any other input method or device that the product or service supports. The easiest way to approach this is to fully document interactions using each input method, and then write procedures that use input-neutral verbs.
Learn about the five types of testing methods used during audit procedures for Type II SOC engagements required to analyze service organization controls. A test method is a method for a test in science or engineering, such as a physical test, chemical test, or statistical test. It is a definitive procedure that produces a test result. In order to ensure accurate and relevant test results, a test method should be "explicit, unambiguous, and experimentally feasible.", as well as effective and
26/10/2009 · Companies often document a process to make sure each worker knows how to perform it correctly, or to analyze a process for improvement. If you are asked to document a process, or decide to do it yourself, make sure you involve people who are experienced and knowledgeable about the process as … procedures. 4. and methods validation. 5. data to support the documentation of the identity, strength, 21 . quality, purity, and potency of drug substances and drug products. 6. It will help you
This document presents a discussion of the characteristics for consideration during the validation of the analytical procedures included as part of registration applications submitted within the EC, Japan and USA. This document does not necessarily seek to cover the testing It provides recommendations on how you, the applicant, can submit analytical procedures and methods validation data to support the documentation of the identity, strength, quality, purity, and
This document presents a discussion of the characteristics for consideration during the validation of the analytical procedures included as part of registration applications submitted within the EC, Japan and USA. This document does not necessarily seek to cover the testing I tried to put in one article all best practices for documenting stored procedures and functions in a relational database. Doing research for this article I found out that despite of those techniques have been here for many years and a lot of people asking questions there aren't many useful resources with compilation of best practices.
Chapter 4. Writing Documentation Procedures and Tools

ISO ISO 164392014 - Information and documentation. The Food and Drug Administration (FDA) is announcing the availability of a draft guidance for industry entitled ``Analytical Procedures and Methods Validation: Chemistry, Manufacturing, and Controls Documentation.'' This draft guidance is intended to provide recommendations to applicants on..., procedures. 4. and methods validation. 5. data to support the documentation of the identity, strength, 21 . quality, purity, and potency of drug substances and drug products. 6. It will help you.
Export documentation and Procedure in Hindi YouTube

Analytical Procedures and Method Validation Highlights of. 17/09/2013 · Entrepreneurs don’t generally like structure, and in your startup or small company, implementing formal written procedures may even seem counterintuitive to … https://en.m.wikipedia.org/wiki/Standard_Operating_Procedures ISO 16439:2014 considers the impact of libraries on individuals, institutions and society. It is applicable to all types of libraries in all countries. However, not all methods described apply to all libraries. Limitations on the applicability of individual methods are specified in the descriptions..

distribution, procedures, systems and record keeping should be reported and investigated for corrective and preventative action (CAPA) Deviation should be documented when there is a deviation from methods or controls in manufacturing documents, material control documents, and/or standard operating procedures. Write your policies and procedures for a wide audience. Time Commitment. Many people want to know how long it takes to document their policies and procedures. Time to produce documentation, of course depends on your knowledge level, writing skills and the amount of material you plan to cover. Generally, for short documents you may want to plan
How to structure quality management system documentation. Author: Ana Meskovska. Usually, when people think of quality management system documentation, they envision loads of documents, and unnecessary and bureaucratic procedures. This is because companies often go overboard when documenting their quality management systems. However, this doesn’t need to be the case. It is true … Standard Operating Procedure: SOURCE DOCUMENTATION Purpose The purpose of this standard operating procedure (SOP) is to provide guidance to research personnel when a system of records is established. Documentation of source data is necessary for the reconstruction, evaluation, and validation of clinical findings, observations, and other activities during a clinical trial. Source documentation
Process Documentation Methods. Most of the methodologies related to process documentation record the program and feed the information to the decision makers and managers so as to make sure that the project decisions are taken in a fast and better way. There are many ways in which process can be documented and few of the most common methods are How to structure quality management system documentation. Author: Ana Meskovska. Usually, when people think of quality management system documentation, they envision loads of documents, and unnecessary and bureaucratic procedures. This is because companies often go overboard when documenting their quality management systems. However, this doesn’t need to be the case. It is true …
Automatic documentation of procedures and methods - To simplify the maintenance or the use of existing code by other developers, WINDEV proposes an automatic documentation for the procedures (or class methods)... distribution, procedures, systems and record keeping should be reported and investigated for corrective and preventative action (CAPA) Deviation should be documented when there is a deviation from methods or controls in manufacturing documents, material control documents, and/or standard operating procedures.
Accounting manuals document the specific policies and procedures a company follows when handling financial information. In the United States, generally accepted accounting principles (GAAP) are the most authoritative accounting standards. GAAP is principles-based, meaning companies have a certain degree of latitude when applying the principles Document control procedures are an integral part of project management. In the absence of clearly established policies and procedures for document control, several problems can arise ranging from mere confusion to grave financial losses. In the following section we will provide you with some tips on how to institute the best document control
ISO 16439:2014 considers the impact of libraries on individuals, institutions and society. It is applicable to all types of libraries in all countries. However, not all methods described apply to all libraries. Limitations on the applicability of individual methods are specified in the descriptions. 17/09/2013 · Entrepreneurs don’t generally like structure, and in your startup or small company, implementing formal written procedures may even seem counterintuitive to …
Process Documentation Methods. Most of the methodologies related to process documentation record the program and feed the information to the decision makers and managers so as to make sure that the project decisions are taken in a fast and better way. There are many ways in which process can be documented and few of the most common methods are Write your policies and procedures for a wide audience. Time Commitment. Many people want to know how long it takes to document their policies and procedures. Time to produce documentation, of course depends on your knowledge level, writing skills and the amount of material you plan to cover. Generally, for short documents you may want to plan
Information and documentation — Methods and procedures for assessing the impact of libraries Information et documentation — Méthodes et procédures pour évaluer l’impact des bibliothèques INTERNATIONAL STANDARD ISO 16439 First edition 2014-04-15 Reference number ISO 16439:2014(E) This is a free 10 page sample. Access the full version DOCUMENT ON TEST METHOD, TESTING EQUIPMENT AND RELATED PROCEDURES FOR OF VEHICLES FOR EMISSION AS PER CMV RULES 115, 116 AND 126 . MoRTH / CMVR / TAP-115/116 (Issue 4) Page 2 CONTENTS Part Chapter Description Page No. Summary of Applicable Emission Norms for Different Catagories of Vehicles and Engines 11 Introduction 12 I -- Details of standards and test procedures for …
16/05/2017 · Explanation of Export Documentation and Procedure in Hindi (हिन्दी में) Analytical Procedures and Methods Validation for Drugs and Biologics. The draft Guidance was reviewed by ISPE members who welcomed the detailed directions on the content of analytical methods. ISPE is pleased to provide the following specific comments intended to add clarity to some areas of the document.
Information and documentation — Methods and procedures for assessing the impact of libraries Information et documentation — Méthodes et procédures pour évaluer l’impact des bibliothèques INTERNATIONAL STANDARD ISO 16439 First edition 2014-04-15 Reference number ISO 16439:2014(E) This is a free 10 page sample. Access the full version This includes mouse, keyboard, voice recognition, game controller, gesture, and any other input method or device that the product or service supports. The easiest way to approach this is to fully document interactions using each input method, and then write procedures that use input-neutral verbs.
Automatic documentation of procedures and methods - To simplify the maintenance or the use of existing code by other developers, WINDEV proposes an automatic documentation for the procedures (or class methods)... And most importantly for a Document Controller to follow this procedure. Here is a document control procedure that I made and submitted to the Engineer. 1. INTRODUCTION. This procedure describes the specific working methods which will be used to carry out the document controlling system in this project. 2. PURPOSE/SCOPE. 2.2 General Description